Table of Contents
AQA | Unit 1 | Chemistry 1
Page 1 | Atoms, periodic table, chemical reactions
Page 2 | Limestone and Building Materials
Page 3 | Metal and their uses
Page 4 | Crude oil and fuels
Page 5 | Other useful substances from crude oil
Page 6 | Plant oils and their uses
Page 7 | Changes in the earth and its atmosphere
AQA | Unit 2 | Chemistry 2
Page 1 | Structure and Bonding
Page 2 | Atomic structure, analysis and quantitative chemistry
Page 3 | Rates of Reactions
Page 4 | Exothermic and Endothermic Reaction
Page 5 | Acids, Bases and Salts
Page 6 | Electrolysis
AQA | Unit 3 | Chemistry 3
Page 1 | The periodic table
Page 2 | Water
Page 3 | Calculating and explaining energy change
Page 4 | Further analysis and quantitative chemistry
Page 5 | The production of ammonia
Page 6 | Alcohols, carboxylic acids and esters
Rates of Reaction
Learning objectives:
to learn how reactions can be measured, and what can affect the speed of reactions
Different reactions occur at different rates. There are different ways to measure the rate of a reaction
⦁ Measure the rate at which the reactant is used up
⦁ Measure the rate at which the product is used up
When measuring the rate of reaction, we can measure many things.
⦁ we can measure the mas of a substance and see how much it changes
⦁ we can measure the volume of gas and see how much Is produced over a period of time
the rate of reaction is measured using this equation
Factors affecting the rate
The rate of reaction can be affected by a number of factors
⦁ Temperature
⦁ Concentration of reactant
⦁ Pressure
⦁ Surface area
⦁ Catalyst
If the temperature increases or decreases then the rate of reaction will change
This graph will illustrate the changes produced by different temperatures
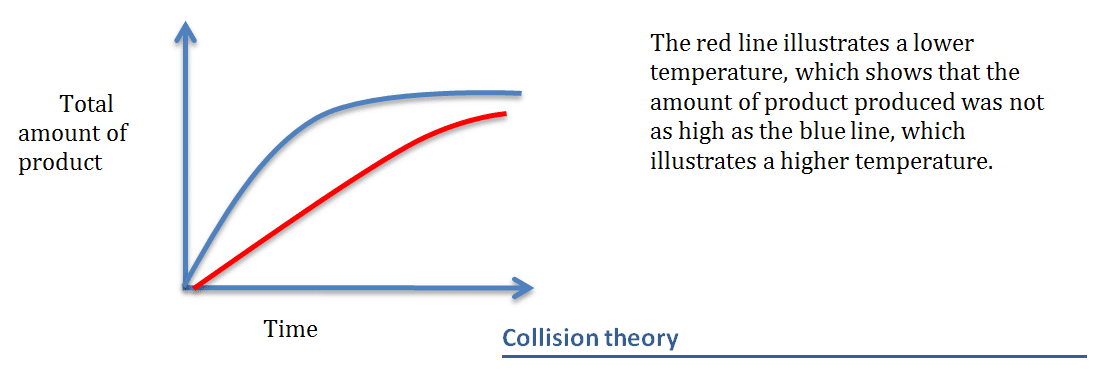
Collision theory
For the chemical reaction to occur the reactant particles must collide. However the collisions must occur with enough energy for the reaction to occur.
Pressure – if the pressure or concentration is increased then there are more particles in a particular volume.
Therefore if the pressure is increases then there is a greater chance of the particles colliding and the rate of the reaction increases.

Surface area – if a solid reactant is broken down into little pieces the surface area increase as there are more particles exposed to the other reactant, therefore increasing the chance of the particles colliding and thus the rate of reaction increases
Changing the temperature – if the temperature is increased, the reactant particles move more quickly and collude with greater energy, therefore they have enough energy to get over the activation energy. The activation energy is the minimum amount of energy required for the reaction to occur.
Using a catalyst – catalyst increase the rate of reaction by lowering the activation energy so more of the collisions are likely to be successful. The key about using a catalyst is that it does not used up in the reaction and remains chemically unchanged.


