Table of Contents
AQA | Unit 1 | Chemistry 1
Page 1 | Atoms, periodic table, chemical reactions
Page 2 | Limestone and Building Materials
Page 3 | Metal and their uses
Page 4 | Crude oil and fuels
Page 5 | Other useful substances from crude oil
Page 6 | Plant oils and their uses
Page 7 | Changes in the earth and its atmosphere
AQA | Unit 2 | Chemistry 2
Page 1 | Structure and Bonding
Page 2 | Atomic structure, analysis and quantitative chemistry
Page 3 | Rates of Reactions
Page 4 | Exothermic and Endothermic Reaction
Page 5 | Acids, Bases and Salts
Page 6 | Electrolysis
AQA | Unit 3 | Chemistry 3
Page 1 | The periodic table
Page 2 | Water
Page 3 | Calculating and explaining energy change
Page 4 | Further analysis and quantitative chemistry
Page 5 | The production of ammonia
Page 6 | Alcohols, carboxylic acids and esters
Electrolysis
Learning objectives:
to learn about electrolysis and how oxidation – reduction reactions occur
During the process of electrolysis, ionic substances are broken down through the use of electricity.
For electrolysis to work the ions must be free to move, this usually occurs when it is dissolved in water or it is molten
During electrolysis the positive ions will move towards the negative electrode and the negative ions will move towards the positive electrode
The positive ions will gain electrons and will be reduced
The negative ions will lose electrons and will be oxidized
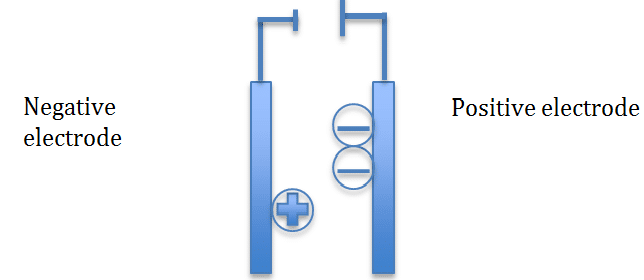
To remember this use this phrase OILRIG (oxidation is loss, reduction is gain)
Electroplating
This is a method used to oat a cheaper metal with a more expensive one.
The negative electrode will be the object you want to be electroplated and the positive electrode will be the supply of that metal. The electrolyte should be a solution of the positive electrode
So for example, if we take a metal spoon and attach it to the negative terminal, it will attract the positive silver ions from the solution. As the number of positive ions is reduced from the solution, it is replaced by positive ions from the positive electrode as it decomposes. So overall the negative electrode will gain ions and eventually the positive electrode will disappear.
Aluminum extraction
One of the main uses for electrolysis is aluminum extraction. Both the electrodes are made from graphite. The aluminum exists in a form called bauxite, which is purified to aluminum oxide. The aluminum oxide is converted to a molten substance so the ions are free to move.
Aluminum will form at the negative electrode and the oxygen will form at the positive electrode.
Predicting the products of electrolysis
At the negative electrode positive ions will gain electrons. But which positive ion will form at the negative electrode is dependent on the reactivity series.
A metal will be produced if it is less reactive than hydrogen and hydrogen will be produced if the metal is more reactive.
The reactivity series is shown below
Potassium
Sodium
Calcium
Magnesium
Aluminum
Carbon
Zinc
Iron
Tin
Lead
Hydrogen
Copper
Silver
Gold
Platinum
At the positive electrode negatively charged ions lose their electrodes so for example chloride will form chlorine.
Half equations
A half equation shows what happens at each electrode
In half equations the electrons are shown as e-
For example
⦁ write out the reactant and product, so for chlorine Cl- Cl2
⦁ balance the equation, so we need two chlorines on both side , 2Cl- Cl2
⦁ make sure each side has the same charge, so the reactants has a minus two, so we need to add two electrons to the products
2Cl- Cl2 + 2e-
in half equations we need to make sure the charges are balanced at the end, and the only way to do that is by adding electrons to the correct side

