Table of Contents
Week 1 | English Grammar
Day 4 |I am doing (present continuous)
Day 5 |Are you doing? (present continuous questions)
Day 6 |I do/work/like (present simple)(present continuous questions)
Week 2 | English Grammar
Day 1 |I don't... (present simple negative)
Day 2 |Do you...? (present simple questions)
Day 3 |I am doing (present continuous) I do (present simple)
Day 4 |I have... and I've got...
Day 6 |Worked/got/went etc (past simple)
Week 3 | English Grammar
Day 1 |I didn't... did you...? (past simple negative and questions)
Day 2 |I was doing (past continuous)
Day 3 |I was doing (past continuous) and I did (past simple)
Day 4 |I have done (present perfect 1)
Day 5 |I've just... I've already... I haven't...yet (present perfect 2)
Day 6 |Have you ever...? (present perfect 3)
Week 4| English Grammar
Day 1 |How long have you...? (present perfect 4)
Day 3 |I have done (present perfect) and I did (past)
Day 4 |Is done, was done (passive 1)
Day 5 |Is being done, has been done (passive 2)
Day 6 |Be/have/do in present and past tenses
Week 5| English Grammar
Day 1 |Regular and irregular verbs
Day 2 |What are you doing tomorrow?
Week 6| English Grammar
Day 2 |Must, mustn't, don't, need to
Day 6 |Do this! Don't do that! Let's do that
Week 7| English Grammar
Day 2 |There is... There are...
Day 3 |There was/were... There has/have been... There will be...
Day 6 |Have you? Are you? Don't you? etc
Week 8| English Grammar
Day 1 |Too/either/so am I/neither do I etc
Day 2 |Isn't/haven't/don't etc (negatives)
Day 3 |Do they? Is it? Have you?
Day 4 |Forming questions (who/what/why/where/when/which)
Day 5 |What...? Which...? How...?
Day 6 |How long does it take...?
Week 9| English Grammar
Day 1 |Do you know where...? I don't know what... etc
Day 2 |He/she said that... He/she told me that...
Day 3 |Work/working Go/going Do/doing
Day 4 |I want you to... I told you to...
Day 5 |I went to the shop to...
Day 6 |Go to... Go on... Go for... Go -ing... Get…
Week 10| English Grammar
Day 4 |I/me He/him They/them etc
Day 6 |Whose is this? It's mine/yours/hers etc
Week 11| English Grammar
Day 1 |Myself/yourself/themselves etc
Week 12| English Grammar
Day 2 |All/most/some/any/no/none etc
Week 13| English Grammar
Day 2 |If we go... if you see... etc
Day 3 |If I had... If we went... etc
Day 4 |A person who... A thing that/which (relative clauses 1)
Introduction to Acids and Alkalis
Acids are liquids which are solutions of compounds in water. For example, hydrochloric acid is a solution of hydrogen chloride. They have a lot of hydrogen ions (H+) in them. Strong acids, or highly concentrated acids, can be corrosive because they’re so reactive, so they must be handled with care.
Alkalis are the solutions of bases, such as sodium hydroxide. A lot of bases are insoluble, therefore, they cannot be alkalis, BUT all alkalis can be bases. They contain hydroxide ions (OH–). Like acids, they can also be corrosive if they’re concentrated, so they must also be handled with care.
While acids and alkalis can be dangerous, they can also be very helpful in our daily lives, if used correctly. For example, you might not know that you’ve been sprinkling ethanoic acid over your fish and chips! (Ethanoic acid is the chemical name for vinegar). Lots of alkalis are used in household cleaning products, like bleach and laundry detergent. Fruits and vegetables also have natural acids in them – especially citric fruit, like lemons and oranges.
| Common examples of acids | Common examples of alkalis |
| Ethanoic acid (Vinegar) | Ammonia |
| Citric acid (Lemon Juice) | Sodium hydroxide |
| Hydrochloric Acid ( used in the lab and found in the stomach) | Magnesium hydroxide (used for indigestion) |
| Carbonic acid (Fizzy drinks) | Sodium bicarbonate (baking soda) |
| Sulphuric Acid | Barium hydroxide |
Properties of acids
- Acids have a pH of less than 7
- They contain hydrogen ions (H+)
- They turn litmus paper to red
- They have a sour taste (DON’T CHECK THIS)
- They can be corrosive or irritant
Properties of alkalis
- Alkalis have a pH of more than 7
- They are dissolved bases
- They contain hydroxide ions (OH–)
- They turn Litmus paper blue
- They have a slippery, soapy feel
- They have a bitter taste (DON’T CHECK THIS)
- They can be corrosive or irritant
Warning Symbols
These are some hazard symbols you may see on the acids and alkalis in the lab. You need to know what they are so that you can act safely around them

This sign means that the chemical is toxic. Anyone who handles this chemical should be wearing safety goggles and gloves, and may need to use a fume cupboard as well.

This sign means that the chemical is an irritant. Anyone handling this should be wearing goggles and if any spills onto their skin, they should wash it off immediately
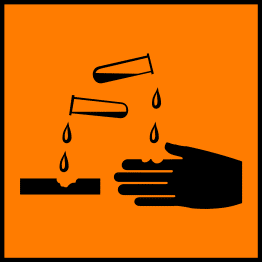
This sign means that the chemical is corrosive. Anyone handling this chemical should protect their eyes with safety goggles and wear gloves.

This sign means that the chemical is highly flammable. In addition to wearing goggles, anyone handling this chemical should take care to keep it away from any open flames or oxidising materials.
KS3 Chemistry Questions– Introduction to Acids and Alkalis


1. What do acids have a high concentration of?
a. Hydroxide ions
b. Hydrogen ions
c. Hydrogen atoms
d. Water
e. Chlorine
2. All acids and alkalis are dangerous
a. True
b. False
3. What roles do alkalis play in our daily lives?
a. Seasoning food
b. Cleaning products
c. Perfume
d. Petrol
4. Which of these naturally contains acid?
a. Sugar
b. Flour
c. Apples
d. Water
e. Wood
5. Which of these is NOT a property of an alkali?
a. Bitter taste
b. Fruity smell
c. pH of more than 7
d. Turn Litmus paper blue
e. Slippery/soapy feel
[bg_collapse view=”link” color=”#fafafa” expand_text=”Reveal Answer” collapse_text=”Hide Answer” inline_css=”background: #2ea3f2; padding: 9px; font-size: 14px; font-weight: 600;” ]
1.b. Hydrogen ions
2.b. False
3.b. Cleaning products
4.c. Apples
5.b. Fruity smell
[/bg_collapse]

1. What is an alkali?
2. Give three examples of common alkali
3. Give three examples of common acids
4. What does![]() mean?
mean?
5. How should a corrosive substance be handled?
[bg_collapse view=”link” color=”#fafafa” expand_text=”Reveal Answer” collapse_text=”Hide Answer” inline_css=”background: #2ea3f2; padding: 9px; font-size: 14px; font-weight: 600;” ]
1.
An alkali is a base which has been dissolved into a liquid.
2.
– Ammonia (could be more answers than this)
– Sodium hydroxide
– Magnesium hydroxide
– Sodium bicarbonate
– Barium hydroxide
3.
– Hydrochloric(stomach) acid (could be more answers than this)
– Citric acid (accept lemon juice)
– Carbonic acid
– Ethanoic acid (accept vinegar)
– Sulphuric acid
– Phosphoric acid
4.This symbol means that the substance is toxic.
5.When handling a corrosive substance, safety goggles should be worn to protect the eyes and gloves to protect the hands.
[/bg_collapse]

